By Ahmed El-Medany
In contrast to their prevalence in the overall disease population, Black individuals were considerably underrepresented in trials that affirmed the United States’ Food and Drug Administration’s (FDA) approval of 24 CVD treatments, researchers reported.A cross-sectional study by Chen and Li evaluated the representation status of Black and White Americans in clinical drug trials by calculating the participation to prevalence ratio (PPR). PPR <0.8 or >1.2 were defined as either underrepresentation or overrepresentation of a particular ethnic group, respectively.
Total enrolment across all trials was 187,294 participants, of whom 2.9% were Black and 83.1% were white. According to the study, the PPR was less than 0.8 for Black participants in every clinical trial. The researchers found that the highest PPR for Black participants was 0.52 for hypertension trials and the lowest PPR was 0.072, observed in hypercholesterolaemia trials.
‘Although substantial efforts have been made to reduce racial inequity in clinical trials participation, previous studies have indicated that the enrolment of Black U.S. participants in CVD trials was still disappointing and that significant racial disparities have persisted over the past 15 years,’ Chen and Li wrote. ‘More effective strategies (eg, a 10% to 50% accrual rate of minority groups in clinical trials) are required to enhance the enrolment of Black participants in CVD clinical trials.’
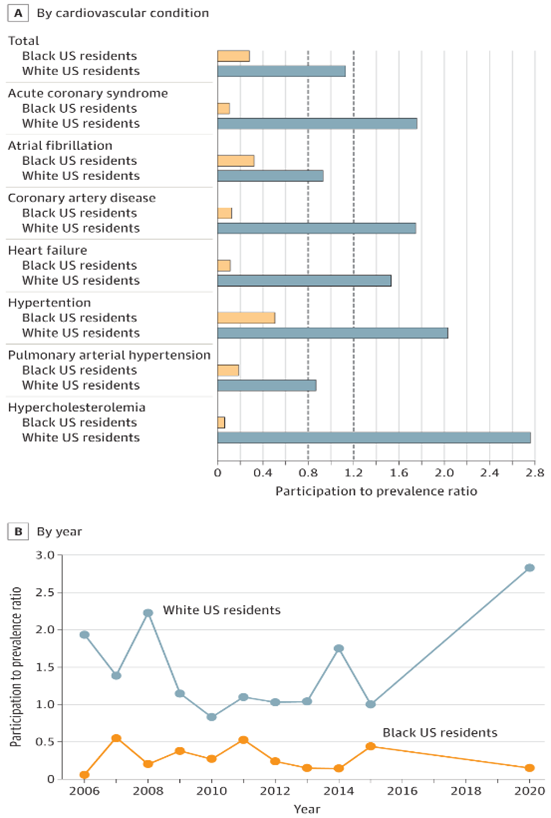
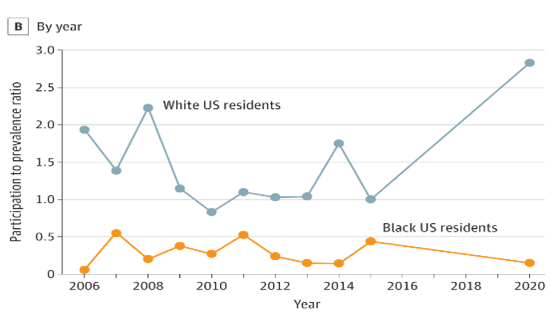
Figure legend: A, The vertical dashed line at participation to prevalence ratio (PPR) of 0.8 indicates that lower values represent underrepresentation, and the dashed line of 1.2 indicates that higher values represent overrepresentation. Values from 0.8 to 1.2 indicate that the proportion of the group in clinical trials nearly equals its proportion in the disease population. B, An annual breakdown shows underrepresentation by Black US residents in cardiovascular drug trials.
Read more at jamanetwork.com
Figure taken from Chen S, Li J. Participation of Black US Residents in Clinical Trials of 24 Cardiovascular Drugs Granted FDA Approval, 2006-2020. JAMA Netw Open. 2021;4(3):e212640. doi:10.1001/jamanetworkopen.2021.2640.