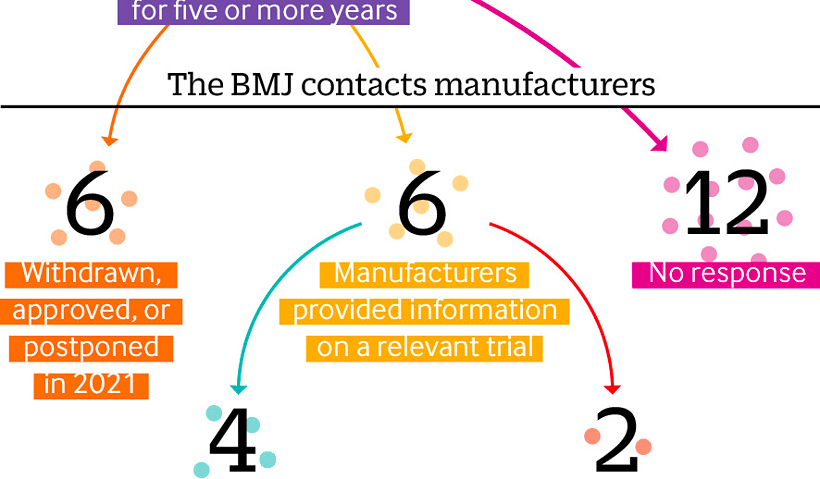
By Elisabeth Mahase Taken Directly from the BMJ doi: https://doi.org/10.1136/bmj.n1898 Criticisms of the US Food and Drug Administration’s accelerated approval process have resurfaced after the recent…
View More FDA allows drugs without proven clinical benefit to languish for years on accelerated pathwayTag: drug safety
NICE publishes updated clinical guideline on the diagnosis and management of atrial fibrillation
Key changes: Bleeding risk scoreNew NICE guideline recommends using the ORBIT bleeding risk score but equally recognises that existing bleeding risk scores may still be…
View More NICE publishes updated clinical guideline on the diagnosis and management of atrial fibrillationLamotrigine may be associated with increased risk of cardiac arrhythmias, FDA warns
By Saadia Aslam An FDA review of study findings showed a potential increased risk of cardiac arrhythmias in patients with pre-existing cardiac disease who are…
View More Lamotrigine may be associated with increased risk of cardiac arrhythmias, FDA warnsConsiderations for prescription of fluoroquinolones in patients developing signs or symptoms of heart valve disease
Dr Laura Dobson comments on a recent update by MHRA regarding fluoroquinolones.
View More Considerations for prescription of fluoroquinolones in patients developing signs or symptoms of heart valve diseaseErythromycin: Caution required due to cardiac risks (QT interval prolongation); drug interaction with rivaroxaban
Drug Safety Update Published 17 December 2020From: Medicines and Healthcare products Regulatory Agency Erythromycin has been associated with events secondary to QT interval prolongation such…
View More Erythromycin: Caution required due to cardiac risks (QT interval prolongation); drug interaction with rivaroxaban