By Ahmed El-Medany
One of the most popular sessions every year, an excellent variety of trials were presented at the 2021 BCS Annual Conference. For those that were unable to attend, or for the sake of refreshing one’s memory, the following is a summary of all the trials presented:
STEP-1
Presented by Dr. Hussain Contractor, perspective by Dr. Mimi Chen
Purpose: Evaluate the efficacy and safety of once-weekly s.c. semaglutide (GLP-1) 2.4mg in people with overweight or obesity, with or without weight-related complications.
Study design and methods:
- Randomised, double blind, placebo-controlled study at 129 sites in 16 countries in Asia, Europe, North America, and South America
- Participants randomised (2:1) to once-weekly s.c. semaglutide 2.4mg (n=1,240) or placebo (n=655) in addition to lifestyle intervention
- Semaglutide initiated at 0.25mg once weekly for the first 4 weeks, escalating every 4 weeks to reach maintenance dose of 2.4mg
- Adherence to lifestyle interventions (reduced calorie diet, increased physical activity) recorded via diary, smartphone app
- Co-primary end-points: % change from baseline to week 68 in body weight, and achievement of body weight reduction ≥5% from baseline at week 68
Results/Conclusion:
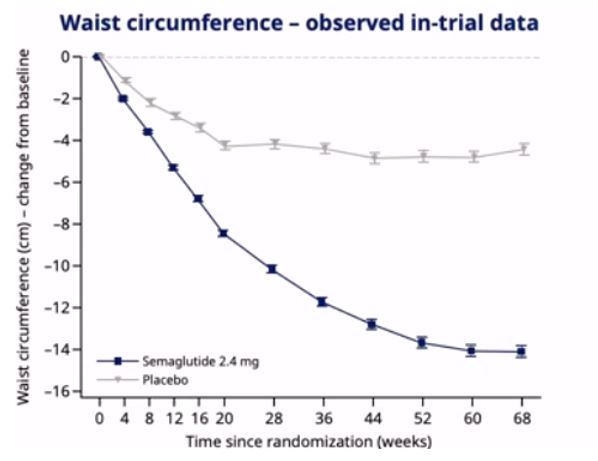
- In adults with overweight or obesity, once-weekly s.c. semaglutide 2.4mg plus lifestyle intervention was associated with substantial, clinically relevant mean weight loss of 16.9% weight reduction, mean -15.3kg, mean BMI -5.5kg/m2, waist circumference -13.5cm
- With 2.4mg, 86.4%, 69.1%, 50.5%, and 32% of participants attained weight loss of >5%, >10%, >15%, and >20%, respectively, by week 68
- Nausea and diarrhoea most common side effects
- Future studies needed to assess longer term outcomes and sustainability, as well as effect on incident diabetes and cardiovascular outcomes. Future studies needed to assess benefit of oral semaglutide
https://www.nejm.org/doi/full/10.1056/NEJMoa2032183
Wilding JP, Batterham RL, Calanna S, Davies M, Van Gaal LF, Lingvay I, McGowan BM, Rosenstock J, Tran MT, Wadden TA, Wharton S. Once-weekly semaglutide in adults with overweight or obesity. New England Journal of Medicine. 2021 Feb 10.
EAST-AFNET4
Presented by Dr. Ben Mercer, perspective by Pro Andre Ng
Purpose: Does early rhythm control lead to better outcomes in contemporaneously treated patients?
Study design and methods:
- International, investigator initiated. 1:1 randomisation to early rhythm control or usual care
- Early rhythm control – anti-arrhythmic drugs (AADs) or catheter ablation as well as direct current cardioversion (DCCV) (Local teams decided as per guidelines)
- Primary outcomes – cardiovascular death, stroke, hospitilisation due to heart failure (HF) or acute coronary syndrome (ACS), number of nights spent in hospital
- N=1,395 early rhythm control. N=1,394 usual care. 5-year follow-up
Results/Conclusion:
- A strategy of early rhythm control in patients with AF <12 months reduced cumulative incidence of death, stroke, HF, and ACS hospitilisation.
- Clinical benefit achieved when combined with evidence-based oral anticoagulation, treatment of concomitant CV conditions, and rate control therapy
- Clinical benefit achieved with use of refined rhythm therapies within guidelines recommendations, and regionally different treatment choices
https://www.nejm.org/doi/full/10.1056/NEJMoa2019422
Kirchhof P, Camm AJ, Goette A, Brandes A, Eckardt L, Elvan A, Fetsch T, van Gelder IC, Haase D, Haegeli LM, Hamann F. Early rhythm-control therapy in patients with atrial fibrillation. New England Journal of Medicine. 2020 Oct 1;383(14):1305-16.
PRAETORIAN
Presented by Dr. Jenny Chuen, perspective by Prof Pier Lambiase
Purpose: Comparison of subcutaneous (S-ICD) and transvenous (T-ICD) systems with regards to device-related complications and inappropriate shocks.
Study design and methods:
- Investigator-initiated randomised controlled non-inferiority trial, funded by Boston Scientific
- Eligibility: Class I or IIa indication for ICD therapy
- Patients randomised 1:1 S-ICD or T-ICD
- Standardised programming: ventricular tachycardia (VT) ~182bpm (+anti-tachycardia pacing (ATP) for T-ICD); ventricular fibrillation (VF) 250bpm
- Primary endpoint: composite of device-related complications and inappropriate shocks
- n=849; median f/u 4 years
Results/Conclusion:
- S-ICD non-inferior to T-ICD in patients with ICD indication (but no indication for pacing) regarding device-related complications and inappropriate shocks, as well as efficacy at terminating malignant arrhythmias (hazard ratio (HR) 0.99; 95% confidence interval (CI) 0.71-1.39; p=0.01)
- Higher incidence of device-related complications in T-ICD balanced by higher risk of inappropriate shocks in S-ICD group
- Limitations: Industry funded. Members of clinical events committee were non-blinded to ICD type. Follow-up period of 4 years too short to provide insight into longer term complications.
https://www.nejm.org/doi/full/10.1056/NEJMoa1915932
Knops RE, Olde Nordkamp LR, Delnoy PP, Boersma LV, Kuschyk J, El-Chami MF, Bonnemeier H, Behr ER, Brouwer TF, Kääb S, Mittal S. Subcutaneous or transvenous defibrillator therapy. New England Journal of Medicine. 2020 Aug 6;383(6):526-36.
EMPEROR-REDUCED
Presented by Dr. Helen Hardy, perspective by Prof Andrew Clark
Purpose: Evaluate benefit of SGLT2 inhibitors in individuals with LVEF <40%, at high risk of HF event.
Study design and methods:
- Double-blind, placebo-controlled, randomised trial
- Eligibility: LVEF <40%, NYHA class II-IV (with or without diabetes)
- 1:1 randomisation. N=1863 received empagliflozin 10mg OD, N=1867 received placebo.
- Primary outcome – composite CV death or hospitilisation for worsening HF.
- Median f/u 16 months
Results/Conclusion:
- In heart failure with reduced ejection fraction (HFrEF), empagliflozin associated with 25% reduction in combined risk of CV death or hospitilisation for HF (primarily driven by 31% reduction in hospitilisation for HF) regardless of whether participants were diabetic or not. Also evidence of slower rate of decline in eGFR in empagliflozin group
- NNT 19 to avoid one event
- Safe and well tolerated: no difference between groups in hypoglycaemia or hypotension. Slight excess of genital infections.
- May 2021 – Empagliflozin approved for use in HFrEF by European Medicines Authority (EMA)
https://www.nejm.org/doi/full/10.1056/NEJMoa2022190
Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, Jamal W. Cardiovascular and renal outcomes with empagliflozin in heart failure. New England Journal of Medicine. 2020 Oct 8;383(15):1413-24.
VICTORIA
Presented by Dr. Joe Cuthbert, perspective by Dr. Ian Squire
Purpose: Evaluate benefit of Vericiguat, an oral guanylate cyclase stimulator, in HFrEF
Study design and methods:
- International multi-centre, double blind, randomised placebo-controlled trial
- Eligibility: LVEF <45%, in previous 12 months NYHA class II-IV
- ‘High risk’ patients identified as having raised BNP in previous month, HF related hospitilisation in previous 6 months, or requirement for outpatient IV diuretic therapy in previous 3 months
- Primary endpoint – first HF hospitilisation or CV death
- N=2524 placebo, n=2526 vericiguat; Median follow-up 10.8 months
Results/Conclusion:
- 10% relative risk reduction in CV mortality or HF hospitilisation. Absolute risk reduction of 4.2 events per 100 patient-years (p=0.02)
- NNT 24 to avoid one event
- Well tolerated in high-risk population
- 60% participants on triple therapy (BB + MRA + ACEi/ARB/ARNi); 32% had CRT +/- ICD
- Data for Entresto and dapagliflozin more compelling; vericiguat unlikely to change UK practice
https://www.nejm.org/doi/full/10.1056/NEJMoa1915928
Armstrong PW, Pieske B, Anstrom KJ, Ezekowitz J, Hernandez AF, Butler J, Lam CS, Ponikowski P, Voors AA, Jia G, McNulty SE. Vericiguat in patients with heart failure and reduced ejection fraction. New England Journal of Medicine. 2020 May 14;382(20):1883-93.
