By Ahmed El-Medany
One of the most popular sessions every year, an excellent variety of trials were presented at the 2021 BCS Annual Conference. For those that were unable to attend, or for the sake of refreshing one’s memory, the following is a summary of all the trials presented:
Ischemia-CKD
Presented by Dr. Mahvash Zaman, prospective provided by Dr. Angela Hoye
Purpose: Assess the effect of revascularisation in stable coronary artery disease in the context of advanced chronic kidney disease (CKD)
Study design and methods:
- Randomised, n=777 with advanced kidney disease and moderate or severe ischaemia on stress testing
- Patients assigned to initial invasive strategy (coronary angiography +/- PCI) in addition to medial therapy; or medial therapy alone (and angiography offered if medical therapy failed)
- Primary outcome: composite of death or nonfatal MI
Results/Conclusion:
- Median follow-up 2.2 years – primary outcome occurred in 123 patients in invasive group and 129 in conservative group (Adjusted hazard ration (HR) 1.01; 95% confidence interval (CI) 0.79-1.29; p=0.95)
- Among patients with coronary artery disease, advanced CKD, and moderate or severe ischaemia, no evidence that initial invasive strategy compared with initial conservative strategy reduced risk of death or nonfatal MI
https://www.nejm.org/doi/full/10.1056/NEJMoa1915925
Bangalore S, Maron DJ, O’Brien SM, Fleg JL, Kretov EI, Briguori C, Kaul U, Reynolds HR, Mazurek T, Sidhu MS, Berger JS. Management of coronary disease in patients with advanced kidney disease. New England Journal of Medicine. 2020 Apr 23;382(17):1608-18.
DEFINITION II
Presented by Dr. Sudhakar George, perspective provided by Dr. Daniel Jones
Purpose: Assess benefit of two-stent techniques for patients with DEFINTION criteria-defined complex coronary bifurcation lesions
Study design and methods:
- N=653, 49 international centres.
- Participants randomly assigned to undergo two-stent technique, or provisional stenting (using a ‘safety’ wire prior to main vessel stenting)
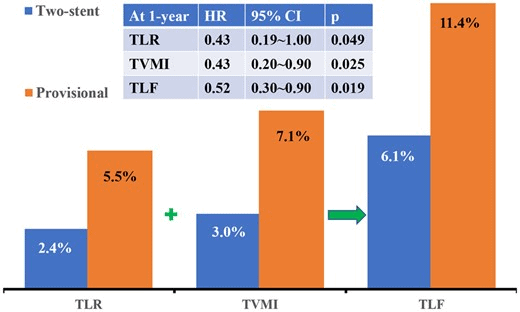
- Primary endpoint: composite of target lesion failure (TLF) at 1 year follow-up, including cardiac death, target vessel MI, and clinically driven target lesion revascularisation (TLR).
Results/Conclusion:
- At 1 year follow-up TLF occurred in 37 (11.4%) vs 20 (6.1%) in provisional and two-stent groups, respectively (HR 0.52; 95% CI 0.3-0.9; p=0.019).
- This was largely driven by target vessel MI – (HR 0.43; 95% CI 0.2-0.9; p=0.025) and clinically driven TLR (HR 0.43; 95% CI 0.19-1.00; p=0.049) in the provisional group.
- Incidence of cardiac death at 1 year was 2.5% and 2.1% in provisional and two-stent group, respectively (HR 0.86; 95% CI 0.31-2.37; p=0.772)
- Two-stent approach associated with significant improvement in clinical outcomes vs provisional stenting approach. Further studies required to identify mechanisms contributing to increased risk of target vessel MI after provisional stenting.
DEFINITON I:
https://www.jacc.org/doi/full/10.1016/j.jcin.2014.04.026
Chen SL, Sheiban I, Xu B, Jepson N, Paiboon C, Zhang JJ, Ye F, Sansoto T, Kwan TW, Lee M, Han YL. Impact of the complexity of bifurcation lesions treated with drug-eluting stents: the DEFINITION study (Definitions and impact of complEx biFurcation lesIons on clinical outcomes after percutaNeous coronary IntervenTIOn using drug-eluting steNts). JACC: Cardiovascular Interventions. 2014 Nov;7(11):1266-76.
DEFINITION 2:
https://academic.oup.com/eurheartj/article/41/27/2523/5862959?login=true
Zhang JJ, Ye F, Xu K, Kan J, Tao L, Santoso T, Munawar M, Tresukosol D, Li L, Sheiban I, Li F. Multicentre, randomized comparison of two-stent and provisional stenting techniques in patients with complex coronary bifurcation lesions: the DEFINITION II trial. European Heart Journal. 2020 Jul 14;41(27):2523-36.
SUCCOUR
Presented by Dr. Arjun Ghosh, perspective provided by Dr. Rebecca Dobson
Purpose: To identify whether global longitudinal strain (GLS) guided cardio-protective therapy (CPT) improves cardiac function of at-risk patients undergoing potential cardiotoxic chemotherapy, compared with usual care.
Study design and methods:
- International multicenter prospective RCT
- N=331 from 23 international sites taking anthracyclines with another risk factor for heart failure (hypertension/diabetes etc.)
- Randomly allocated into 166 undergoing GLS-guided CPT (CPT provided if >12% relative reduction in GLS using Echopac software) and 165 ejection fraction (EF)-guided CPT (CPT provided if >10% absolute reduction of EF).
Results/Conclusion:
- N=307 followed-up after 1 year. 9 (5.8%) met criteria for chemotherapy-related cardiac dysfunction (CTCRD) in the GLS-guided arm vs 22 (14.3%) in the EF-guided arm (p=0.02).
- Therefore, the incidence of CTCRD was reduced by strain-guided cardioprotection. However, the final EF and the number of patients developing new LV dysfunction (EF <55%) was not altered by GLS-guided CPT. Results support the use of GLS in surveillance for CTRCD.
https://academic.oup.com/eurheartj/article/41/Supplement_2/ehaa946.3282/6004273?login=true
Negishi T, Thavendiranathan P, Penicka M, Lemieux J, Aakhus S, Miyazaki S, Shirazi M, Galderisi M, Cho GY, Popescu BA, Kosmala W. Cardioprotection using strain-guided management of potentially cardiotoxic cancer therapy: 1 year results of the SUCCOUR trial. European Heart Journal. 2020 Nov;41(Supplement_2):ehaa946-3282.
RECOVERY
Presented by Dr. Sveeta Badiani, perspective by Dr. Guy Lloyd
Purpose: Evaluate benefit of glucocorticoids in modulating inflammation-mediated lung injury and reducing progression to respiratory failure and death.
Study design and methods:
- Controlled, open-label trial. N=2,104 randomly assigned to receive dexamethasone for up to 10 days and 4,321 to receive usual care.
- Primary outcome: 28-day mortality
Results/Conclusion:
- 482 (22.9%) in the dexamethasone group and 1,110 (25.7%) in the usual care group died within 28 days after randomisation (age-adjusted rate ratio, 0.83; 95% CI 0.75-0.93; p<0.001)
- In steroid group, incidence of death was lower than that in the usual care group among patients receiving invasive mechanical ventilation (29.3% vs. 41.4%; rate ratio, 0.64; 95% CI, 0.51 to 0.81) and among those receiving oxygen without invasive mechanical ventilation (23.3% vs. 26.2%; rate ratio, 0.82; 95% CI, 0.72 to 0.94) but not among those who were receiving no respiratory support at randomization (17.8% vs. 14.0%; rate ratio, 1.19; 95% CI, 0.92 to 1.55).
- Dexamethasone reduced 28-day mortality in hospitalised patients with COVID-19 in those receiving invasive mechanical ventilation or oxygen alone at randomisation, but not in those receiving no respiratory support.
https://www.nejm.org/doi/full/10.1056/NEJMoa2021436
RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19. New England Journal of Medicine. 2021 Feb 25;384(8):693-704.
LoDoCo2
Presented by Dr. Hussain Contractor, perspective by Prof Colin Berry
Purpose: Evaluate whether colchicine reduces the risk of cardiovascular events in patients with chronic coronary artery disease (CAD).
Study design and methods:
- Randomised, controlled, double-blind trial. N=5,522
- Patients with chronic CAD assigned to 0.5mg colchicine (n=2,762) OD or placebo (n=2,760). Median follow-up 28.6 months
- Primary endpoint: composite of cardiovascular death, spontaneous (nonprocedural) MI, ischaemic stroke, or ischaemia-driven coronary revascularisation
Results/Conclusion:
- Primary endpoint occurred in 187 (6.8%) in colchicine group vs 264 (9.6%) in placebo group (HR 0.69, 95% CI 0.57-0.83, p<0.001)
- Incidence of death from non-cardiovascular causes higher in colchicine group (HR 1.51, 95% CI 0.99-2.31)
- Risk of cardiovascular events significantly lower among those receiving 0.5mg colchicine OD vs placebo
