Taken directly from European Heart Journal
Many cardiac pacemakers and defibrillators are not approved by regulators for magnetic resonance imaging (MRI) which makes patient access to MRI challenging, but there is no evidence of increased clinical risk. It is estimated that patients have a 50–75% probability of requiring an MRI during the lifetime of the device.This study showed there was no increased risk of MRI in patients with non-MR conditional pacemaker or defibrillator leads when following recommended protocols.
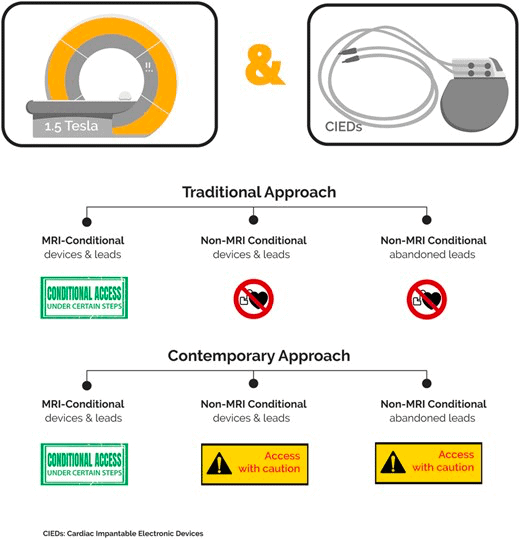
- Patients undergoing clinical 1.5T MRI with pacemakers and defibrillators in three centres over 5 years were included.
- Magnetic resonance imaging protocols were similar for MR-conditional and non-MR conditional systems.
- Devices were interrogated pre- and immediately post-scan, and at follow-up, and adverse clinical events recorded.
- Lead parameter changes peri-scan were stratified by MR-conditional labelling.
- A total of 1148 MRI examinations were performed in 970 patients (54% non-MR conditional systems, 39% defibrillators, 15% pacing-dependent) with 2268 leads.
- There were no lead-related adverse clinical events, and no clinically significant immediate or late lead parameter changes following MRI in either MR-conditional or non-MR conditional leads.
- Small reductions in atrial and right ventricular sensed amplitudes and impedances were similar between groups, with no difference in the proportion of leads with parameter changes greater than pre-defined thresholds (7.1%, 95% confidence interval: 6.1–8.3).
- The study also included 40 patients with abandoned leads, these patients were often excluded in the past.
Standardising MR conditions for all leads would significantly improve access to MRI by enabling patients to be scanned in non-specialist centres, with no discernible incremental risk.
Read full article here:
https://academic.oup.com/eurheartj/advance-article/doi/10.1093/eurheartj/ehab350/6357859
Read related editorial here:
https://academic.oup.com/eurheartj/advance-article/doi/10.1093/eurheartj/ehab469/6362538
References:
Anish N Bhuva, Russell Moralee, Tamara Brunker, Karen Lascelles, Lizette Cash, Kush P Patel, et al. Evidence to support magnetic resonance conditional labelling of all pacemaker and defibrillator leads in patients with cardiac implantable electronic devices, European Heart Journal, 2021;, ehab350, https://doi.org/10.1093/eurheartj/ehab350
Chiara Bucciarelli-Ducci, Panos Vardas, Reconsidering safety and reducing barriers to MRI in patients with cardiac implantable electronic devices, European Heart Journal, 2021;, ehab469, https://doi.org/10.1093/eurheartj/ehab469
